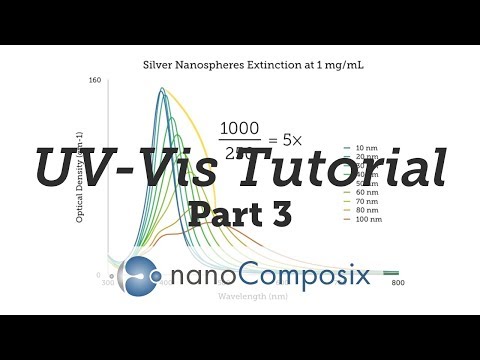
Hi there, welcome to part 3 in our demonstrational UV-Vis video series. In this installment, I'll discuss how to analyze your measurement data so you can calculate absorbance and mass concentration. I'll also show you examples of overlaid spectra for comparison studies and explain what to look for. Hope you enjoy the video! So, in part 2, we went through how to prepare an analytical or qualitative UV-Vis measurement. Now that we have that data, there are a few things we can do with it. First, we need to correct our absorbance data to take the dilution into account. For example, let's say that to get a peak optical density of about 1, you had taken 1 mL of your sample and diluted it in 99 mL of water. That's a 100-fold dilution. If, on the UV-Vis, you measured the peak absorbance at 1, you would multiply that by 100, which gives you a dilution-corrected peak absorbance value of 100. You can use Excel to multiply this number on all of your measurement values. We recommend you use the analytical measurement method we described in part 2 in order to calculate the most accurate dilution factor instead of relying on your pipette volumes. Here at Nanocomposites, we've put together a spreadsheet that does all the calculating for us and automatically generates charts for every UV-Vis dataset that gets entered. We have a simplified template that we would like to share. It's available for download on the website by visiting this webpage. Once you're into the raw dataset and input your nanoparticle sample volume and total volume after dilution, the spreadsheet will have everything it needs to generate the UV-Vis spectrum and stats. Here's a chart where we've overlaid spectra from three different sizes of silver. These spectra are normalized, which means we took the spectrum...
Award-winning PDF software





Video instructions and help with filling out and completing Are Form 8453 S Calculator